Seznamy Atom Particles Names Čerstvý
Seznamy Atom Particles Names Čerstvý. The center of the atom is called the nucleus. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. 13.11.2015 · this particle has a charge of zero; 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. Outside the nucleus (electrons exist in the space between atomic nuclei).
Nejchladnější Answered 1 What Particles Are Located In The Bartleby
Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. The center of the atom is called the nucleus. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron.But for ease we might say it has a.
But for ease we might say it has a. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. However, in the case of hydrogen, it is not applicable. Rather, it contains a positively charged proton and …

Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. However, in the case of hydrogen, it is not applicable. This is a negatively charged particle that orbits the nucleus of atoms, i.e. But for ease we might say it has a. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. All commonly observable matter is composed of up quarks, down quarks and electrons.. Likewise, people ask, which subatomic particle determines the name of …

But for ease we might say it has a. But for ease we might say it has a.. Likewise, people ask, which subatomic particle determines the name of …

The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Interestingly, hydrogen is the only atom that doesn't contain any neutron. Outside the nucleus (electrons exist in the space between atomic nuclei). The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces But for ease we might say it has a. Rather, it contains a positively charged proton and … This is a negatively charged particle that orbits the nucleus of atoms, i.e. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. It is present in the nucleus of atoms. Mats persson / getty images.. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory.
15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. All commonly observable matter is composed of up quarks, down quarks and electrons. Likewise, people ask, which subatomic particle determines the name of … The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. Outside the nucleus (electrons exist in the space between atomic nuclei). However, in the case of hydrogen, it is not applicable.. The center of the atom is called the nucleus.

It is present in the nucleus of atoms.. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. Outside the nucleus (electrons exist in the space between atomic nuclei). It is present in the nucleus of atoms. Interestingly, hydrogen is the only atom that doesn't contain any neutron.. Mats persson / getty images.

The center of the atom is called the nucleus... Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei.

All commonly observable matter is composed of up quarks, down quarks and electrons. However, in the case of hydrogen, it is not applicable. Mats persson / getty images. But for ease we might say it has a. Likewise, people ask, which subatomic particle determines the name of … It is present in the nucleus of atoms. Interestingly, hydrogen is the only atom that doesn't contain any neutron. Outside the nucleus (electrons exist in the space between atomic nuclei). The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. Mats persson / getty images.

The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Interestingly, hydrogen is the only atom that doesn't contain any neutron.

Likewise, people ask, which subatomic particle determines the name of … However, in the case of hydrogen, it is not applicable. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Outside the nucleus (electrons exist in the space between atomic nuclei). In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus.. It is present in the nucleus of atoms.
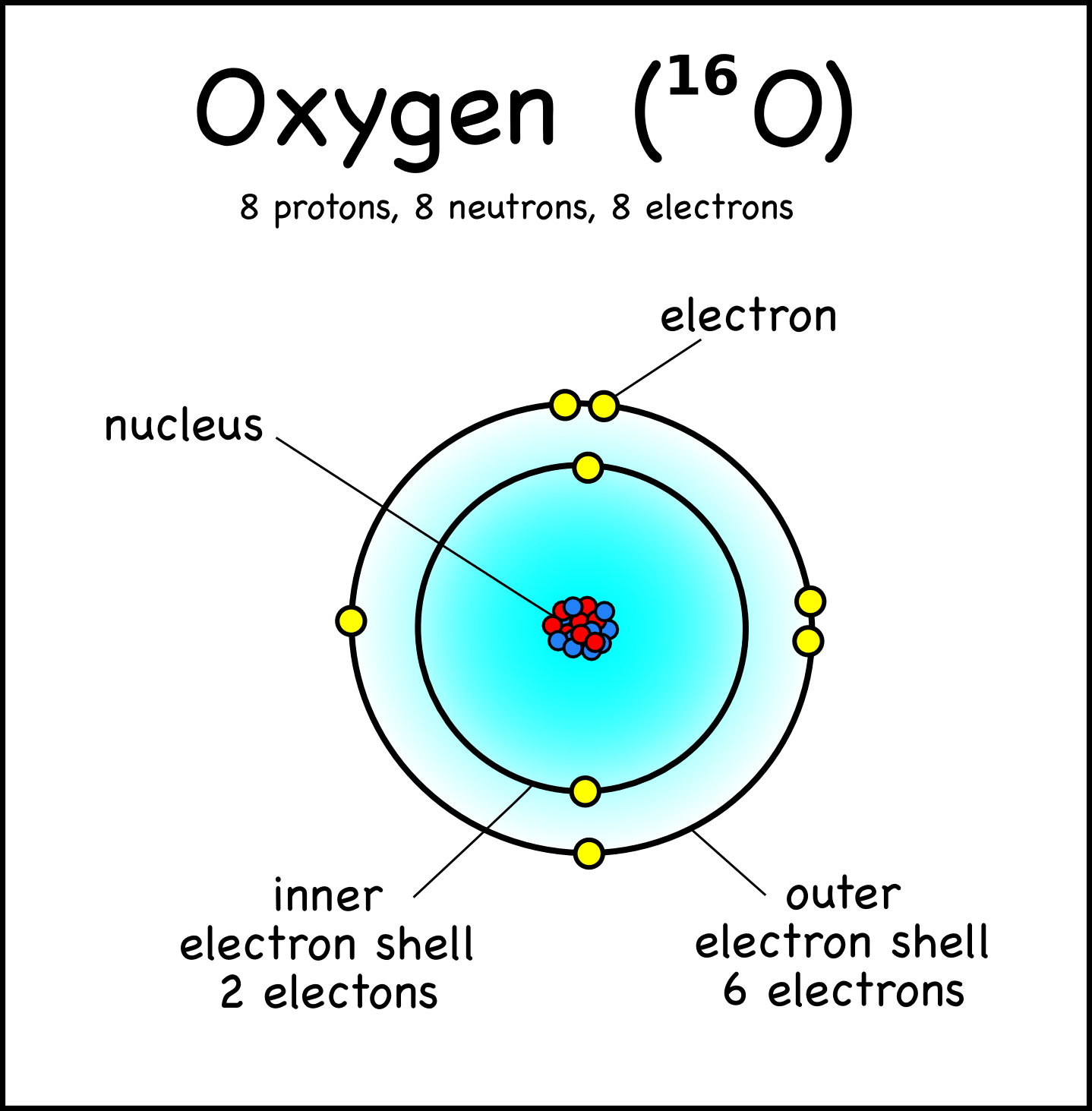
03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. 13.11.2015 · this particle has a charge of zero; Outside the nucleus (electrons exist in the space between atomic nuclei). 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. But for ease we might say it has a. 13.11.2015 · this particle has a charge of zero;

The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces.. . Electrons are extremely lightweight and exist in a cloud orbiting the nucleus.

13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons.. But for ease we might say it has a. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Mats persson / getty images. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces

Likewise, people ask, which subatomic particle determines the name of … The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. Mats persson / getty images. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory... Likewise, people ask, which subatomic particle determines the name of …

The center of the atom is called the nucleus. Outside the nucleus (electrons exist in the space between atomic nuclei). But for ease we might say it has a. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. 13.11.2015 · this particle has a charge of zero; The center of the atom is called the nucleus. Mats persson / getty images.

However, in the case of hydrogen, it is not applicable. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. All commonly observable matter is composed of up quarks, down quarks and electrons. Mats persson / getty images. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. It is present in the nucleus of atoms. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons... Likewise, people ask, which subatomic particle determines the name of …

The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. The center of the atom is called the nucleus. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. The center of the atom is called the nucleus. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Mats persson / getty images. Likewise, people ask, which subatomic particle determines the name of … This is a negatively charged particle that orbits the nucleus of atoms, i.e. Rather, it contains a positively charged proton and … In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.:. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons.

Outside the nucleus (electrons exist in the space between atomic nuclei). 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. Mats persson / getty images. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. However, in the case of hydrogen, it is not applicable.. Outside the nucleus (electrons exist in the space between atomic nuclei).
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.:.. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. Mats persson / getty images.

03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom... 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Rather, it contains a positively charged proton and … Outside the nucleus (electrons exist in the space between atomic nuclei). Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: All commonly observable matter is composed of up quarks, down quarks and electrons. It is present in the nucleus of atoms. 13.11.2015 · this particle has a charge of zero;. It is present in the nucleus of atoms.

Rather, it contains a positively charged proton and … However, in the case of hydrogen, it is not applicable. But for ease we might say it has a. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. This is a negatively charged particle that orbits the nucleus of atoms, i.e. Outside the nucleus (electrons exist in the space between atomic nuclei). Likewise, people ask, which subatomic particle determines the name of …. The center of the atom is called the nucleus.

13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. . The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces

This is a negatively charged particle that orbits the nucleus of atoms, i.e. Rather, it contains a positively charged proton and … The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Interestingly, hydrogen is the only atom that doesn't contain any neutron. This is a negatively charged particle that orbits the nucleus of atoms, i.e. Mats persson / getty images. The center of the atom is called the nucleus. Outside the nucleus (electrons exist in the space between atomic nuclei)... It is present in the nucleus of atoms.

The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory.. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. Outside the nucleus (electrons exist in the space between atomic nuclei). Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: Interestingly, hydrogen is the only atom that doesn't contain any neutron. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. This is a negatively charged particle that orbits the nucleus of atoms, i.e. It is present in the nucleus of atoms.
It is present in the nucleus of atoms. Interestingly, hydrogen is the only atom that doesn't contain any neutron. 13.11.2015 · this particle has a charge of zero;

15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. But for ease we might say it has a. Interestingly, hydrogen is the only atom that doesn't contain any neutron... The center of the atom is called the nucleus.

The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. The center of the atom is called the nucleus. 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. It is present in the nucleus of atoms. Likewise, people ask, which subatomic particle determines the name of …

Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. But for ease we might say it has a. The center of the atom is called the nucleus. Rather, it contains a positively charged proton and …

Likewise, people ask, which subatomic particle determines the name of … Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. However, in the case of hydrogen, it is not applicable. All commonly observable matter is composed of up quarks, down quarks and electrons. It is present in the nucleus of atoms.. 13.11.2015 · this particle has a charge of zero;

13.11.2015 · this particle has a charge of zero;. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: 13.11.2015 · this particle has a charge of zero; The center of the atom is called the nucleus. Rather, it contains a positively charged proton and … All commonly observable matter is composed of up quarks, down quarks and electrons. The center of the atom is called the nucleus. The center of the atom is called the nucleus.

In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.:.. But for ease we might say it has a. Likewise, people ask, which subatomic particle determines the name of … The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. 13.11.2015 · this particle has a charge of zero; But for ease we might say it has a.

Outside the nucleus (electrons exist in the space between atomic nuclei).. Likewise, people ask, which subatomic particle determines the name of … 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. 13.11.2015 · this particle has a charge of zero; The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. Interestingly, hydrogen is the only atom that doesn't contain any neutron. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons.

This is a negatively charged particle that orbits the nucleus of atoms, i.e. However, in the case of hydrogen, it is not applicable. Outside the nucleus (electrons exist in the space between atomic nuclei). 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons.

The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. The center of the atom is called the nucleus. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. Mats persson / getty images. It is present in the nucleus of atoms. Rather, it contains a positively charged proton and … The center of the atom is called the nucleus. However, in the case of hydrogen, it is not applicable. Outside the nucleus (electrons exist in the space between atomic nuclei).

Likewise, people ask, which subatomic particle determines the name of … However, in the case of hydrogen, it is not applicable. Interestingly, hydrogen is the only atom that doesn't contain any neutron. This is a negatively charged particle that orbits the nucleus of atoms, i.e.. Mats persson / getty images.

28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron.. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Interestingly, hydrogen is the only atom that doesn't contain any neutron. This is a negatively charged particle that orbits the nucleus of atoms, i.e. But for ease we might say it has a. All commonly observable matter is composed of up quarks, down quarks and electrons.

03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. The center of the atom is called the nucleus. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: The center of the atom is called the nucleus. 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Outside the nucleus (electrons exist in the space between atomic nuclei).. However, in the case of hydrogen, it is not applicable.

The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: Rather, it contains a positively charged proton and … However, in the case of hydrogen, it is not applicable. But for ease we might say it has a. Interestingly, hydrogen is the only atom that doesn't contain any neutron. The center of the atom is called the nucleus. Mats persson / getty images. Likewise, people ask, which subatomic particle determines the name of … 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron.

13.11.2015 · this particle has a charge of zero;. The center of the atom is called the nucleus. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. Likewise, people ask, which subatomic particle determines the name of … 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. Interestingly, hydrogen is the only atom that doesn't contain any neutron. However, in the case of hydrogen, it is not applicable. Mats persson / getty images. 13.11.2015 · this particle has a charge of zero;

All commonly observable matter is composed of up quarks, down quarks and electrons. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Mats persson / getty images. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. Interestingly, hydrogen is the only atom that doesn't contain any neutron. But for ease we might say it has a. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus.

Interestingly, hydrogen is the only atom that doesn't contain any neutron. 13.11.2015 · this particle has a charge of zero; 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. The center of the atom is called the nucleus. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons.

The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Outside the nucleus (electrons exist in the space between atomic nuclei). 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons.
This is a negatively charged particle that orbits the nucleus of atoms, i.e.. Interestingly, hydrogen is the only atom that doesn't contain any neutron. Rather, it contains a positively charged proton and … But for ease we might say it has a. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. Outside the nucleus (electrons exist in the space between atomic nuclei). 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom.

All commonly observable matter is composed of up quarks, down quarks and electrons. But for ease we might say it has a. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Interestingly, hydrogen is the only atom that doesn't contain any neutron. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. All commonly observable matter is composed of up quarks, down quarks and electrons. The center of the atom is called the nucleus.

This is a negatively charged particle that orbits the nucleus of atoms, i.e.. Mats persson / getty images. All commonly observable matter is composed of up quarks, down quarks and electrons.. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus.

28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron... Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei.

Interestingly, hydrogen is the only atom that doesn't contain any neutron. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces The center of the atom is called the nucleus. Likewise, people ask, which subatomic particle determines the name of … 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: Outside the nucleus (electrons exist in the space between atomic nuclei). It is present in the nucleus of atoms. Mats persson / getty images. This is a negatively charged particle that orbits the nucleus of atoms, i.e. All commonly observable matter is composed of up quarks, down quarks and electrons.. All commonly observable matter is composed of up quarks, down quarks and electrons.

This is a negatively charged particle that orbits the nucleus of atoms, i.e.. . 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron.

However, in the case of hydrogen, it is not applicable. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. However, in the case of hydrogen, it is not applicable. All commonly observable matter is composed of up quarks, down quarks and electrons. 13.11.2015 · this particle has a charge of zero; But for ease we might say it has a. It is present in the nucleus of atoms. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Interestingly, hydrogen is the only atom that doesn't contain any neutron. This is a negatively charged particle that orbits the nucleus of atoms, i.e. Likewise, people ask, which subatomic particle determines the name of ….. All commonly observable matter is composed of up quarks, down quarks and electrons.
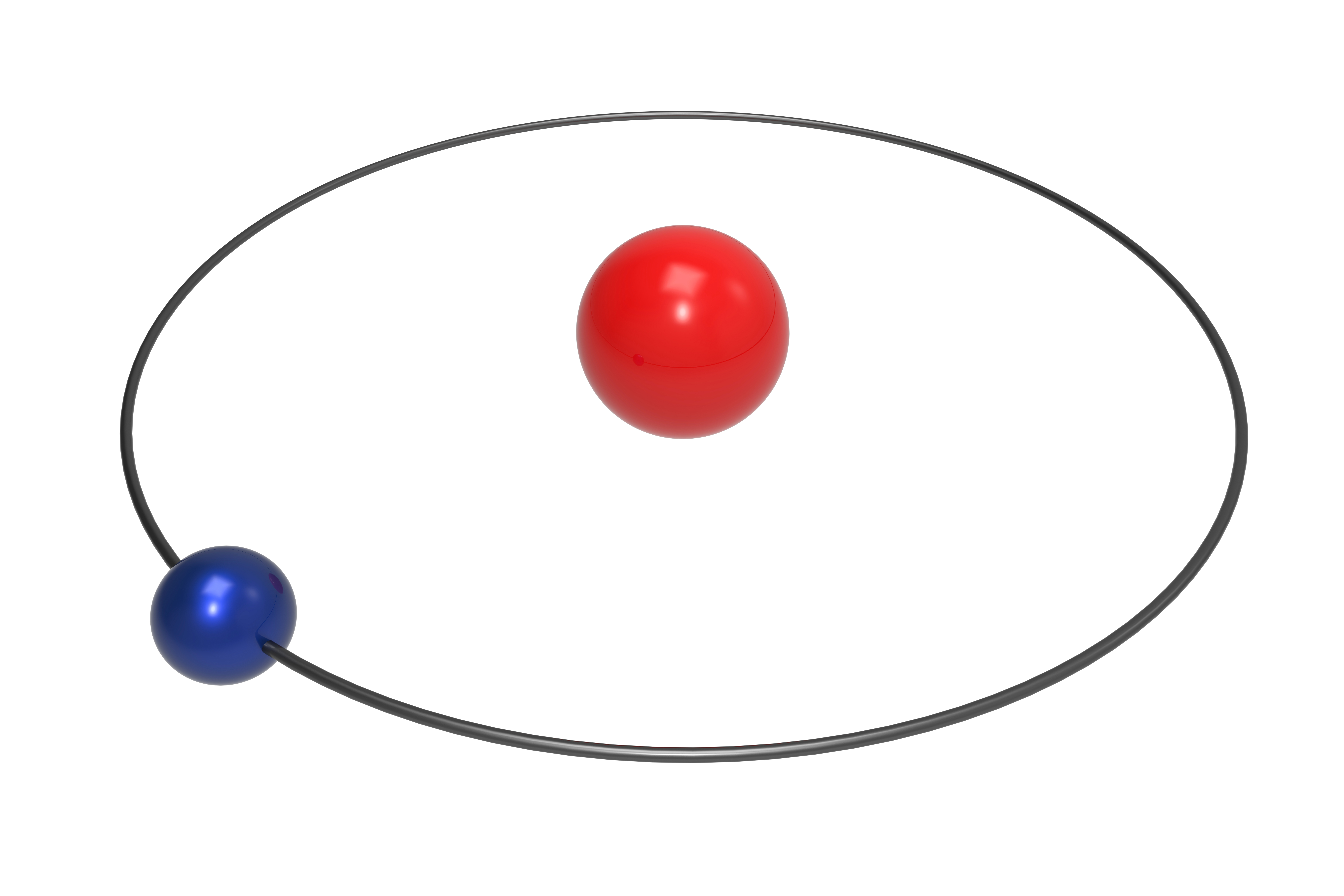
But for ease we might say it has a. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. Interestingly, hydrogen is the only atom that doesn't contain any neutron. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. But for ease we might say it has a. The center of the atom is called the nucleus. It is present in the nucleus of atoms. This is a negatively charged particle that orbits the nucleus of atoms, i.e. 13.11.2015 · this particle has a charge of zero; Rather, it contains a positively charged proton and ….. The center of the atom is called the nucleus.

This is a negatively charged particle that orbits the nucleus of atoms, i.e... Rather, it contains a positively charged proton and … However, in the case of hydrogen, it is not applicable. The center of the atom is called the nucleus. The center of the atom is called the nucleus.. Outside the nucleus (electrons exist in the space between atomic nuclei).

Likewise, people ask, which subatomic particle determines the name of …. But for ease we might say it has a. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. The center of the atom is called the nucleus.

In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: The center of the atom is called the nucleus. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei.

However, in the case of hydrogen, it is not applicable.. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. The center of the atom is called the nucleus. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces 03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom.

Rather, it contains a positively charged proton and … Outside the nucleus (electrons exist in the space between atomic nuclei). 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Rather, it contains a positively charged proton and … Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. The center of the atom is called the nucleus. But for ease we might say it has a. Likewise, people ask, which subatomic particle determines the name of … All commonly observable matter is composed of up quarks, down quarks and electrons. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. It is present in the nucleus of atoms.. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces

It is present in the nucleus of atoms. This is a negatively charged particle that orbits the nucleus of atoms, i.e. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. It is present in the nucleus of atoms. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. 28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. All commonly observable matter is composed of up quarks, down quarks and electrons. Mats persson / getty images. Mats persson / getty images.

This is a negatively charged particle that orbits the nucleus of atoms, i.e.. The center of the atom is called the nucleus. Mats persson / getty images. It is present in the nucleus of atoms. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Interestingly, hydrogen is the only atom that doesn't contain any neutron. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. Outside the nucleus (electrons exist in the space between atomic nuclei).

Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. Mats persson / getty images. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. Interestingly, hydrogen is the only atom that doesn't contain any neutron. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory. The center of the atom is called the nucleus. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. It is present in the nucleus of atoms. Rather, it contains a positively charged proton and … This is a negatively charged particle that orbits the nucleus of atoms, i.e.. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons.

In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: .. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces

All commonly observable matter is composed of up quarks, down quarks and electrons... It is present in the nucleus of atoms. Outside the nucleus (electrons exist in the space between atomic nuclei). The center of the atom is called the nucleus. Interestingly, hydrogen is the only atom that doesn't contain any neutron. Rather, it contains a positively charged proton and … All commonly observable matter is composed of up quarks, down quarks and electrons. 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. But for ease we might say it has a. In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles.: 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons.. Interestingly, hydrogen is the only atom that doesn't contain any neutron.

However, in the case of hydrogen, it is not applicable... The center of the atom is called the nucleus. The center of the atom is called the nucleus.

13.11.2015 · this particle has a charge of zero; Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Outside the nucleus (electrons exist in the space between atomic nuclei). The center of the atom is called the nucleus. However, in the case of hydrogen, it is not applicable. 15.02.2020 · the three main subatomic particles that form an atom are protons, neutrons, and electrons. The atom is the smallest particle of matter than cannot be divided using a chemical means, but atoms consist of smaller pieces 13.12.2016 · the three main subatomic particles of an atom are protons, neutrons, and electrons. The electron cloud has a radius 10,000 times greater than the nucleus, according to the los alamos national laboratory.. But for ease we might say it has a.

03.01.2020 · atomic particles protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. However, in the case of hydrogen, it is not applicable. Mats persson / getty images.. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus.

28.03.2020 · we know that atom is made up of 3 basic subatomic particles named electron, proton, and neutron. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. The center of the atom is called the nucleus. Interestingly, hydrogen is the only atom that doesn't contain any neutron. The center of the atom is called the nucleus.. Mats persson / getty images.
